The Doctor phoned the duty Microbiologist to chat things through and the Microbiologist explained that “Bilharzia” not only affects the bladder but some species can cause intestinal infection too, so it was a possibility. They agreed that the Doctor would arrange for a number of stool samples to be sent to the microbiology laboratory as well as a blood test to look for infection. The Doctor wasn’t convinced but the patient was happy for the thoroughness of the investigation.
Bilharzia is the common name for schistosomiasis, no not a new dog breed (clearly I’ve been watching too much of the BBC’s series “Pooch Perfect!”), but a parasitic infection caused by flukes that infect blood vessels. Bilharzia is named after Theodor Bilharz, a German doctor who first identified the parasitic cause way back in 1852 but who unfortunately later died, at the age of only 37, from complications of another waterborne-illness, typhoid, caught in Egypt!
There are five main species of Schistosome that infect humans in different areas of the World, although the first 3 are the more common:
- Schistosoma haematobium – Africa and the Middle East
- Schistosoma mansoni – Africa and South America
- Schistosoma japonicum – East Asia
- Schistosoma intercalatum – West and Central Africa
- Schistosoma mekongi – Cambodia and Laos
Schistosomiasis is more common in rural areas than in urban centres because the life cycle of the parasite requires fresh water snails and these are much less likely to be found in towns and cities. Risk of infection is higher in lakes, rivers and estuaries rather than open water as the snails live on grasses and reads at the banks of the water sources.
Schistosomiasis is endemic in many countries but few within these populations have a high enough burden of parasites to be symptomatic or turn into “patients”. In the UK most cases of schistosomiasis are associated with travel to endemic countries, especially Africa.
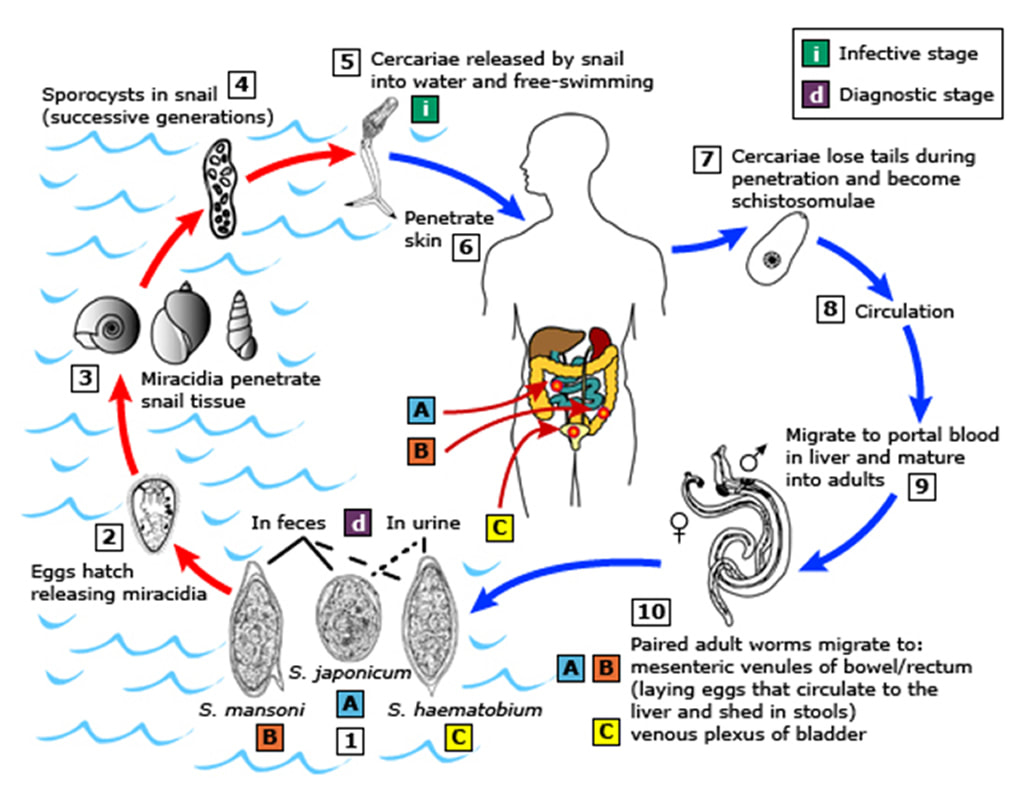
The life cycle of Schistosoma spp.
Each of the Schistosoma spp. has a specific snail species which they require for their life cycle to be completed:
- S. mansoni – Biomphalaria spp.
- S. haematobium and S. intercalatum – Bulinus spp.
- S. japonicum – Oncomelania spp.
- S. mekongi – Tricula spp.
The life cycle:
Parasite eggs are excreted into freshwater from the infected faeces of a human (or animal). These eggs hatch and the microscopic larvae penetrate into the freshwater snails where they undergo further maturation before being released back into the water 4-6 weeks later as infectious cercariae; they remain infectious for up to 2 days but they are more infectious when just released. The cercariae then penetrate through the skin, especially if it is waterlogged like “pruned feet in the bath”, of another unsuspecting human (or animal) where it migrates, via the bloodstream, to the liver. Here they mature into adults over the next 2-4 weeks. The adults then swim against the blood flow to reach the blood vessels supplying the intestines or bladder depending on the species of parasite. In the blood vessels male and female worms mate followed by the female producing eggs which penetrate out into the intestine or bladder and the cycle repeats itself. The adult female, is bigger than the male, at around 7-20 mm long… Yep that’s up to 2cm long… its huge… so yes, you would be able to see it without a microscope! Added to this horror, adult worms usually live for 5-7 years but have been known to survive for 30 years or more!
S. haematobium primarily infects blood vessels supplying the bladder whereas the other Schistosoma spp. infect blood vessels supplying the bowel. Symptoms are therefore related to where the infection is happening in the body. Most infections are asymptomatic, especially in people from endemic countries who have developed immunity to the parasite and therefore do not get the hypersensitivity reactions which are characteristic of this infection.
The initial infection in schistosomiasis is known as “swimmer’s itch” because when the parasite penetrates through the swimmers skin it can cause a dermatitis reaction; this localised immune reaction only occurs with repeat exposure as it requires priming however you can still develop the later signs of infection without having had “swimmers itch”.
Katayama fever is the name given to the later acute hypersensitivity reaction to schistosomiasis occurring 3-8 weeks after the initial infection. It is caused by an immune response to the parasites within the blood stream. The symptoms of Katayama fever include sudden onset of fever, urticaria and angioedema, chills, myalgias, arthralgia, cough, diarrhoea, abdominal pain, and headache.
The most significant consequences of schistosomiasis are from chronic infections and are worse with high numbers of locally trapped eggs, longer duration of symptoms and a strong immune response:
- Intestinal – weight loss, iron deficiency anaemia, colonic ulceration, polyps and dysplasia (early cancer) and occasionally strictures and bowel obstruction
- Hepatosplenic – occlusion of portal veins causes portal hypertension and splenomegaly followed by the development of gastrointestinal varices and the possibility of variceal bleeding
- Pulmonary – occurs especially with hepatosplenic chronic infection where eggs bypass the liver to embolise the lungs causing pulmonary hypertension and cor pulmonale (heart failure due to pulmonary hypertension)
- Genitourinary – bladder fibrosis and calcification leading to urethral strictures, obstruction and renal failure as well as bladder cancer
- Neurological – worms can occasionally enter blood vessels to the spinal cord and brain causing nerve damage, cerebral lesions and encephalopathy
How is schistosomiasis diagnosed?
A clinical history suggesting possible exposure is the key to diagnosis in non-endemic countries like the UK. Patients with bladder schistosomiasis may have terminal haematuria (bleeding at the end of passing urine… this is not life-threatening bleeding as its name might suggest!)
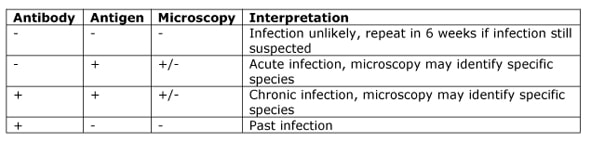
The two main methods for diagnosing schistosomiasis are serology and microscopy.
Serology tests detect either antibodies or antigens. The antibody test is useful in returned travellers and becomes positive 6-12 weeks after infection however it doesn’t distinguish between past or current infection and so is of little use in patients from endemic countries. The antigen tests are of more use in that they detect active infection and can be additionally helpful as they become negative again 5-10 days after successful treatment.
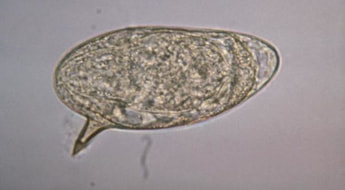
Microscopy can be performed on urine or stool depending on the species of interest. Each type of Schistosome spp. has a characteristic appearance, usually dependent on size, shape and the location of the operculum (the little pointy bit that sticks out from the egg and makes it look like a “speech bubble”).
How is schistosomiasis treated?
The mainstay of treatment of schistosomiasis is the drug Praziquantel. Praziquantel works by damaging the surface of the worm as well as causing it to become paralysed. Praziquantel is not active against larvae so the infection has to have been present for at least 4-6 weeks before it will be effective. NB The damage to the surface of the worm can cause more antigens to leak out and worsen the hypersensitivity reaction so in acute infection it is essential to give steroids prior to Praziquantel to first dampen down this reaction.
Chronic schistosomiasis is treated with Praziquantel without steroids. The dose is dependent on the species:
S. haematobium S. mansoni S. intercalatum |
PO Praziquantel 40mg/kg stat and repeat at 2 weeks |
S. japonicum S. mekongi |
PO Praziquantel 60mg/kg stat and repeat at 2 weeks |
Attempts to prevent schistosomiasis are undertaken in endemic countries by trying to ensure clean water supplies, safe disposal of faeces and regular mass treatment of the population to reduce the amount of environmental contamination.
So our patient’s blood tests confirmed that she did indeed have active schistosomiasis and the laboratory where able to identify S. mansoni. She was treated with Praziquantel and her symptoms settled over the next few weeks. Her repeat tests all showed successful treatment and everyone was pleased; the patient was better, the Microbiologist had a good story for his next teaching session and the Doctor, although he had considered doing an antigen test on himself too, decided he’d just jot it down as a great case for his CPD portfolio…

 RSS Feed
RSS Feed
