How is Antibiotic Resistance Tested in the Laboratory?
Antibiotic resistance is determined using four methods in the laboratory:
Bacterial Species Identification
Each bacteria has different patterns of sensitivity and resistance to the array of antibiotics available. Once a bacteria has been identified (Gram stain, ZN stain etc) resistance patterns can be implied, as certain bacteria are known to be consistently resistant to certain antibiotics (see section – Antibiotics, What is Antibiotic Resistance?)
- Implication of resistance from bacterial species identification
- Disc diffusion using, for example, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) method
- Measurement of the minimum inhibitory concentration (MIC)
- Measurement of the minimum bactericidal concentration (MBC)
Bacterial Species Identification
Each bacteria has different patterns of sensitivity and resistance to the array of antibiotics available. Once a bacteria has been identified (Gram stain, ZN stain etc) resistance patterns can be implied, as certain bacteria are known to be consistently resistant to certain antibiotics (see section – Antibiotics, What is Antibiotic Resistance?)
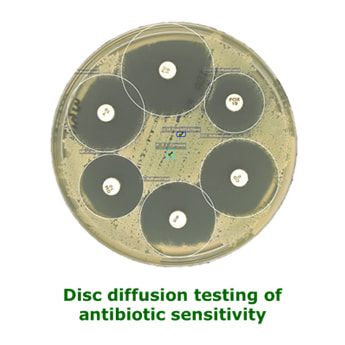
Disc Diffusion
Antibiotic-impregnated filter paper discs are placed on specific agar plates, which have been inoculated with the bacteria to be tested. If the bacteria are sensitive to the antibiotic they will not be able to grow in a zone around the antibiotic, called the zone of inhibition. Resistant bacteria will be able to grow close to the disc. Because resistance is usually relative it is necessary to measure the zone diameter to see if it is large enough to correspond to physiologically achievable concentrations of antibiotic. EUCAST publish regular updates to their method including the zone sizes for bacteria and antibiotic combinations. This method takes 24-48 hours.
Antibiotic-impregnated filter paper discs are placed on specific agar plates, which have been inoculated with the bacteria to be tested. If the bacteria are sensitive to the antibiotic they will not be able to grow in a zone around the antibiotic, called the zone of inhibition. Resistant bacteria will be able to grow close to the disc. Because resistance is usually relative it is necessary to measure the zone diameter to see if it is large enough to correspond to physiologically achievable concentrations of antibiotic. EUCAST publish regular updates to their method including the zone sizes for bacteria and antibiotic combinations. This method takes 24-48 hours.
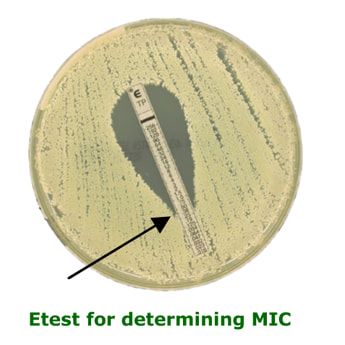
Minimum Inhibitory Concentration (MIC)
The MIC is the least amount of antibiotic required to prevent a bacterium from multiplying. The bacterium may still be alive. It is only usually performed in specific clinical scenarios under the instruction of a Microbiologist, e.g. infective endocarditis. The most common method employed in most UK laboratories is the Etest method whereby a strip impregnated with an antibiotic gradient is placed on an inoculated agar plate. The MIC is determined by how far up the strip the bacterium can grow. Low concentrations (bottom of strip) allow growth whereas higher concentrations (top of strip) inhibit the growth. The MIC is the point at which the growth meets the strip. This method takes 24-48 hours.
The MIC is the least amount of antibiotic required to prevent a bacterium from multiplying. The bacterium may still be alive. It is only usually performed in specific clinical scenarios under the instruction of a Microbiologist, e.g. infective endocarditis. The most common method employed in most UK laboratories is the Etest method whereby a strip impregnated with an antibiotic gradient is placed on an inoculated agar plate. The MIC is determined by how far up the strip the bacterium can grow. Low concentrations (bottom of strip) allow growth whereas higher concentrations (top of strip) inhibit the growth. The MIC is the point at which the growth meets the strip. This method takes 24-48 hours.
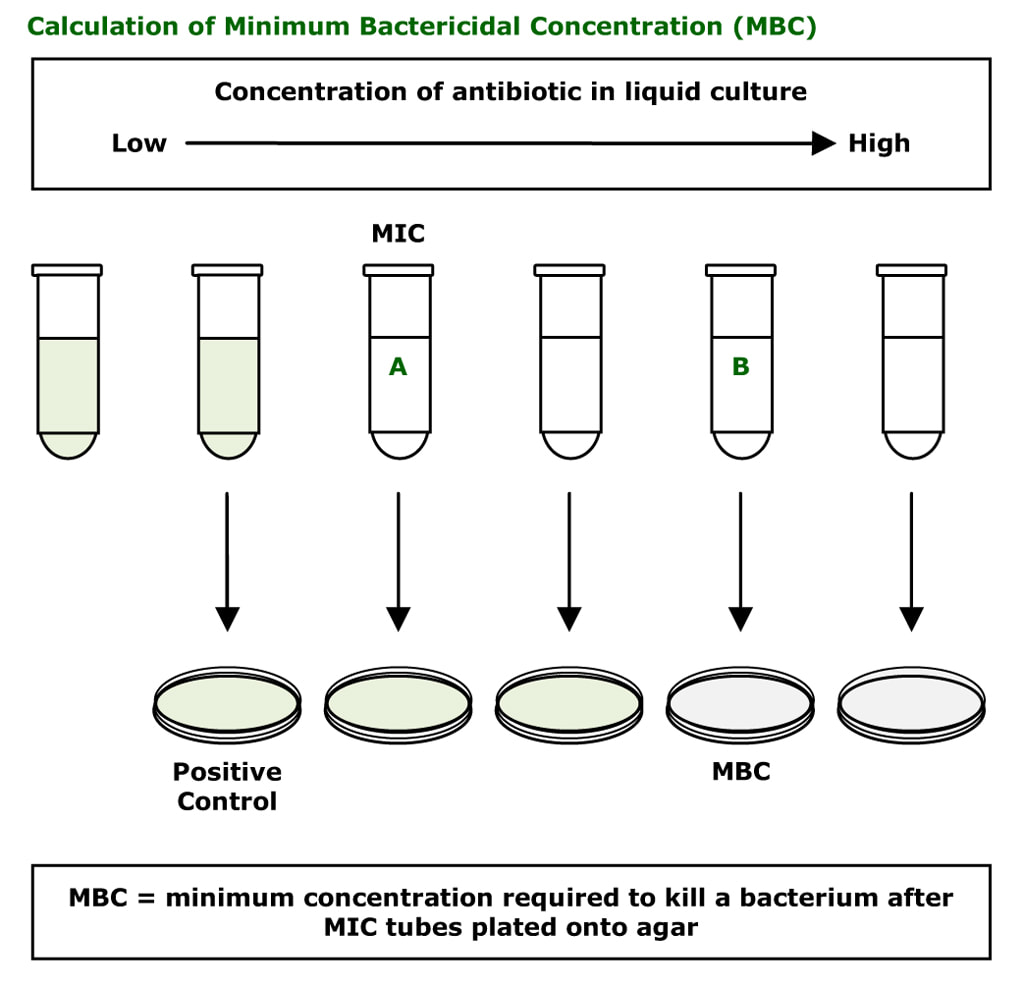
Minimum Bactericidal Concentration (MBC)
The MBC is the least amount of antibiotic required to kill a bacterium. It is very rarely performed. It is difficult to do and labour intensive. Different dilutions of antibiotic are prepared in liquid culture media from low concentration to high concentration. The bacterium is then inoculated into these tubes. After 24-48 hours the tubes where the bacterium is growing become cloudy (green tubes in the diagram below); some tubes show no bacterial growth (clear tubes in the diagram below).
This test allows the laboratory to initially determine the MIC (the lowest concentration of antibiotic required to prevent a bacterium from multiplying). The first clear tube shows inhibition of growth and corresponds to the MIC.
The MBC is determined by plating out the liquid cultures to agar. The first cloudy tube is known to have the bacterium growing and is used as a positive control, while the clear tubes have either inhibited or killed bacteria in them. The agar does not contain antibiotic therefore any living bacteria will now not be inhibited and start to grow (e.g. tube A). Tube B is the MBC; the bacterium in the tube has not grown on the agar because it has been killed by the concentration of antibiotic that was in tube B.
This test allows the laboratory to initially determine the MIC (the lowest concentration of antibiotic required to prevent a bacterium from multiplying). The first clear tube shows inhibition of growth and corresponds to the MIC.
The MBC is determined by plating out the liquid cultures to agar. The first cloudy tube is known to have the bacterium growing and is used as a positive control, while the clear tubes have either inhibited or killed bacteria in them. The agar does not contain antibiotic therefore any living bacteria will now not be inhibited and start to grow (e.g. tube A). Tube B is the MBC; the bacterium in the tube has not grown on the agar because it has been killed by the concentration of antibiotic that was in tube B.

Topics in Antibiotics:
All these topics are covered in the book...Ready to buy your copy? Click here to buy your copy of "Microbiology Nuts & Bolts" Its updated and amazingly only slightly larger considering its got 1/3 more in it! (11cmx18cmx2.5cm).
- Antimicrobial Stewardship
- How Antibiotics Work - Mechanisms of Action
- How to Choose an Antibiotic
- Prophylaxis vs. Treatment
- How to Prescribe an Antibiotic
- The Daily Review of Antibiotic Therapy
- Reasons for Failing Antibiotic Therapy
- Intravenous to Oral Switching of Antibiotics
- Therapeutic Drug Monitoring (TDM)
- Interpretation of TDM
- Antibiotic Dosing in Adult Renal Impairment
- Adjustment of Antibiotic Doses in Adult Renal Impairment
- Antibiotic Dosing in Obesity
- What is Antibiotic Resistance?
- How Resistance Occurs - Mechanisms of Resistance
- How is Antibiotic Resistance Spread?
- How is Antibiotic Resistance Detected in the Laboratory?
- Table of Antibiotic Spectrum of Activity
- Table of Antibiotic Tissue Penetration
- Allergy to Beta-Lactam Antibiotics
- Including pages on each: Penicillins, Cephalosporins, Carbapenems and Aztreonam, Trimethoprim and Co-Trimoxazole (Septrin), Erythromycin, Clarithromycin, Azithromycin and Clindamycin, Gentamicin, Amikacin and Tobramycin, Ciprofloxacin and Levofloxacin, Vancomycin and Teicoplanin, Daptomycin, Metronidazole, Doxycycline, Tigecycline and Tetracycline, Linezoli, Rifampicin, Fusidic Acid, Colistin, Chloramphenicol, Nitrofurantoin, Fidaxomicin, Fosfomycin, Antimycobacterials, Antifungals and Antivirals
All these topics are covered in the book...Ready to buy your copy? Click here to buy your copy of "Microbiology Nuts & Bolts" Its updated and amazingly only slightly larger considering its got 1/3 more in it! (11cmx18cmx2.5cm).