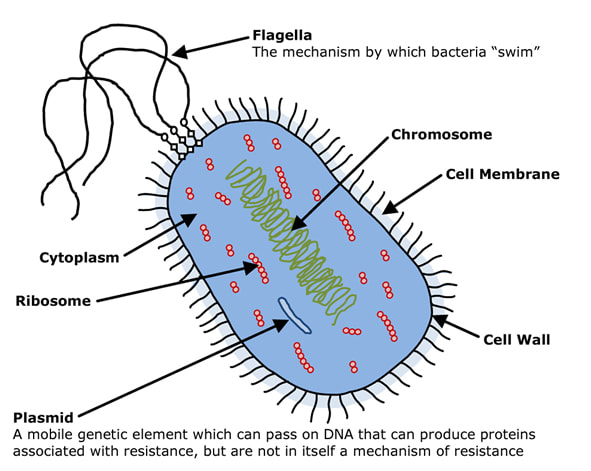
Proteus mirabilis is a Gram-negative bacillus. It grows as a facultative anaerobe (a poor term as it means it grows both aerobically and anaerobically). It is one of the family of gut bacteria known as the enterobacteriaceae or by its slang term “coliforms”.
In the modern era of MaldiTOF the identification of P. mirabilis involves “stuffing it through a machine” and the answer comes out the other end but it is good to remember how it is done for when a “The Machine” is not available or it has broken down.
The first stage in the identification of P. mirabilis is getting it to grow as single colonies on agar. P. mirabilis has an annoying habit of swarming over blood agar and covering the whole plate in an amorphous mass of bacteria, swamping everything else that might have grown on the plate. Nuts & Bolts was described as overcoming a swarm of Proteus in a peer review! “In the Proteus-like swarm of handbooks this one has found its niche...it still has a place in my bag several weeks after I first picked it up” (The British Society for Antimicrobial Chemotherapy). We thought that was quite clever!
CLED is commonly used in the laboratory to indicate whether a bacterium ferments the sugar lactose to produce an acid (lactic acid) and hence a colour change in the agar from blue to yellow. However P. mirabilis is a non-lactose fermenter (E. coli and Klebsiella spp. are common lactose fermenters) but the lack of electrolytes in CLED inhibits the function of the bacterial flagellum meaning that the bacterium cannot move across a surface. CLED has been specifically designed to stop bacteria swarming.
There is another distinctive characteristic of P. mirabilis… IT STINKS! I have been in microbiology since 2001 and I have got used to most smelly things; very little puts me off my food these days… but the smell of P. mirabilis does it every time. It is said to have a very fishy smell but take it from me, that fish has been lying dead washed up on a beach in the sun for several days! Urrgggghhhh… just writing about it makes me feel funny….
So we now have a Gram-negative bacillus growing as a facultative anaerobe which has swarmed over the blood agar but produced single colonies of a non-lactose fermenter on CLED… AND IT STINKS! This is normally enough to identify P. mirabilis but if you need to know for sure and the MaldiTOF is still not working then you could to do further biochemical tests.
In the good old days this meant setting up an API strip of various biochemical reactions which when read and scored gave a number which could be put in a database to give the bacterium a name. In the even better old days we used to set up individual tubes of different biochemical reactions and then consulted the “oracle of all things microbiological” also known as Cowan and Steel's Manual for the Identification of Medical Bacteria! This book contained hundreds of tables of different biochemical reactions of bacteria which allowed you to work out what your bacterium was… it was time consuming but “good fun”… honest…
For P. mirabilis the key reactions were hydrogen sulphide (H2S) production, indole negative, urease positive.
So there you have it, the identification of P. mirabilis without using MaldiTOF.
What is the clinical significance of P. mirabilis?
So having grown it, and identified it, what does the presence of P. mirabilis mean to the patient?
The main site of infection with P. mirabilis by far is the urinary tract. P. mirabilis is part of the normal flora of the gastrointestinal (GI) tract however the human body has a bit of a design flaw. The exit from the GI tract is very close to the exit from the urinary tract and any bacteria living in the GI tract, that can swim, can travel up into the urinary tract to potentially cause a UTI. Bacteria capable of causing UTIs also need to have another mechanism to help them successfully cause UTIs; they need adhesins (a surface component or secretion e.g. protein or polysaccharide) to allow them to recognise and stick to the cells of the urinary tract. P. mirabilis has this ability.
P. mirabilis has another ability which makes it a particular problem when it causes UTIs. Remember back when I has discussing the old fashioned way of identifying P. mirabilis using biochemical reactions? Well one of those reactions was its ability to produce urease. In fact P. mirabilis produces a very strong urease.
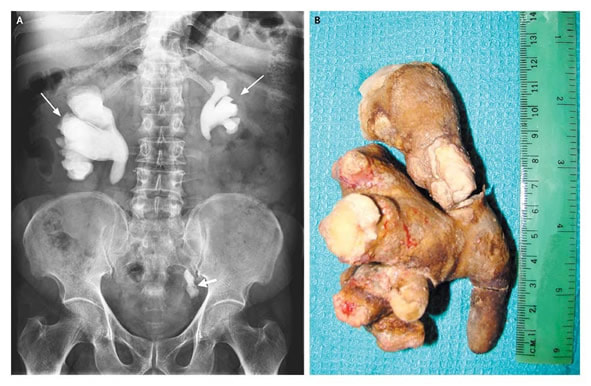
Urease is an enzyme that breaks down urea (CO(NH2)2) in the presence of water to ammonia (NH3) and carbon dioxide (CO2). Whilst urine is normally slightly acidic, ammonia is very alkaline and therefore the breakdown of urea to ammonia and carbon dioxide, alkalinise the urine. This in turn allows salts in urine to precipitate out to form renal, ureteric and bladder stones, some of which take the shape of the inside of the kidney called “staghorn calculi”. These stones in turn can cause obstruction to urine flow leading to renal failure as well as being incredibly painful when they get stuck on the way out (female patients have told me this renal colic is the second most severe pain they have ever had, only labour is worse… which makes it the most severe pain in men then…). Not all patients with P. mirabilis UTIs have stones but it is something to think about when you discover it in a sample.
How is infection with P. mirabilis treated?
There are two parts to the treatment of infection with P. mirabilis; give antibiotics and remove the focus.
Lots of antibiotics are naturally active against P. mirabilis but there is some inherent resistance and acquired resistance is becoming an increasing problem.
Of the antibiotics commonly used to treat UTIs the biggest problem is Nitrofurantoin which has no activity against P. mirabilis. Nitrofurantoin is a pro-drug activated by an enzyme in the bacteria known as nitrofuran reductase, and P. mirabilis does not have this enzyme therefore in P. mirabilis UTIs Nitrofurantoin is never activated. P. mirabilis is also resistant to Colistin (polymyxin E) although this is rarely used to treat patients with UTIs except under the guidance of a Microbiologist who hopefully will remember it’s not active!
Like all of the enterobacteriaceae (e.g. E. coli, Klebsiella spp., Enterobacter spp., etc.) P. mirabilis can acquire new resistance mechanisms on mobile genetic elements such as plasmids. Of particular concern is the increasing prevalence of extended-spectrum beta-lactamases (ESBL) and carbapenemases (CPE).
Whatever antibiotic is chosen, if there is an underlying problem such as a stone, catheter or stent it should ideally be removed, but this is often easier said than done. It is relatively easy to change a urinary catheter but removing a stone or infected stent may require an invasive procedure or even an open operation and some patients are just too frail to undergo this type of surgery. In this case patients are sometimes given a long-term antibiotic to try and prevent further severe infections and positive blood cultures. However this can drive further antibiotic resistance, yet this is a risk many doctors and patients are willing to take, including myself if there are really no other options.
So we plated the blood cultures on to CLED agar and incubated it overnight. The next day there were two different colony types growing on the agar as discrete colonies; the CLED had stopped the P. mirabilis swarming. Feeling somewhat pleased with myself I started to talk about how the P. mirabilis could be identified using its growth characteristics and biochemical reactions… yet again I was met with a look that suggested I was crazy… “okay, okay” I conceded… “stick it through that new-fangled gadget called MaldiTOF in order to get your answer if you want.” The BMS just smiled and opened the lid of the agar plate making me beat a hasty retreat as a plume of rancid fishy-ness escaped into the lab. I’ll just wait until the MaldiTOF isn’t working and then I’ll get my chance to shine…

 RSS Feed
RSS Feed
